Lo Loestrin Fe is the only available low-dose birth control pill with 10 mcg of daily estrogen.
FOR PREGNANCY PREVENTION∗
THERE’S A LOT TO LOVE ABOUT LO LOESTRIN® FE
*If you are moderately obese, discuss with your healthcare provider whether Lo Loestrin Fe is appropriate for you.
*If you are moderately obese, discuss with your healthcare provider whether Lo Loestrin Fe is appropriate for you.
THERE’S ONLY ONE LO LOESTRIN FE
A birth control pill is considered low dose if it contains 35 micrograms (mcg) of estrogen or less.

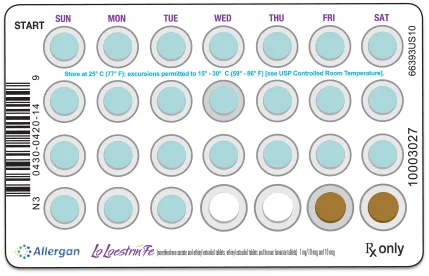
LO LOESTRIN FE IS AN EXTENDED-REGIMEN BIRTH CONTROL PILL
It has 26 days of “active pills” (with hormones) and 2 days of “reminder pills” (without hormones).

WHAT'S IN
EACH PILL
1 milligram
norethindrone
acetate (progestin)
and
10 micrograms
ethinyl estradiol
(estrogen)

10 micrograms
ethinyl estradiol
(estrogen)

75 milligrams
ferrous fumarate
(iron)


WHEN TO
START LO LO
NEW TO HORMONAL
BIRTH CONTROL?
Your healthcare provider may tell you to start Lo Loestrin Fe on the first day of your period. This is the day you start bleeding or spotting (even if it is almost midnight when the bleeding begins).
You should take a Lo Loestrin Fe pill every day until the pack is empty. Then you start a new pill pack.
TAKING LO LOESTRIN FE:
WHAT TO EXPECT AND WHEN
Your body will need time to adjust to Lo Loestrin Fe. Here’s a sneak peek at what to expect when starting–and staying–on Lo Loestrin Fe.

PLAY IT SAFE
Start Lo Loestrin Fe on the first day of your period. If you start on any other day, use a back-up method of birth control–like a condom and spermicide–for the first 7 days.
Pro tip: Lo Loestrin Fe does not protect against STDs, so make it a habit to use a condom every time you have sex.
FIRST
WEEKS
.png)

BLEEDING AND SPOTTING
It’s not uncommon to have some bleeding and spotting between periods. This is sometimes called “breakthrough” bleeding and usually occurs during the first few months of use. However, about one-third of women who use Lo Loestrin Fe had breakthrough bleeding that continued after one year of use.
If the breakthrough bleeding is heavy or lasts for more than a few days, you should discuss this with your healthcare provider.
CONTINUING
LO LO


YOU MAY SEE A CHANGE
IN YOUR PERIODS
While taking Lo Loestrin Fe for pregnancy prevention, most women had a period that lasted LESS THAN 2 DAYS per cycle, on average.

Some women experienced periods that were
LIGHTER THAN NORMAL.


MISS A PERIOD? IT HAPPENS
It’s not uncommon to miss a period while on birth control pills.
In a clinical study, about half of women missed a period after their first year (cycle 13) on Lo Loestrin Fe.

Call your healthcare provider if you go two or more months in a row without a period, or you miss your period after a month when you did not take all your pills correctly.
Notify your healthcare provider if you have symptoms of pregnancy, such as morning sickness or unusual breast tenderness. Stop taking Lo Loestrin Fe if you are pregnant.


